Rapid On-Site Evaluation (R.O.S.E)
For fine needle aspirations or even fine needle biopsies, you may be concerned about specimen adequacy, appropriate triage of specimens (for culture or flow cytometry) or even a real time diagnosis (malignant versus benign). Rapid On-Site Evaluation can answer all of these questions. Given the literal and figurative costs of repeat samples, a ROSE can save you both time and money.
ROSE has been performed for evaluation of transbronchial and percutaneous FNA lung samples, thyroid and pancreas lesions and lymph nodes (sentinel, suspicious and for staging) in both the adult and pediatric populations.
When ROSE is used fewer passes for EBUS (Endoscopic ultrasound) FNA of endobronchial samples are required and it decreases the need for repeat FNAs of pancreatic samples.
In this section we detail the minimal equipment for ROSE procedures, the procedure itself and the benefits of adding on the R.O.S.E to your next evaluation.
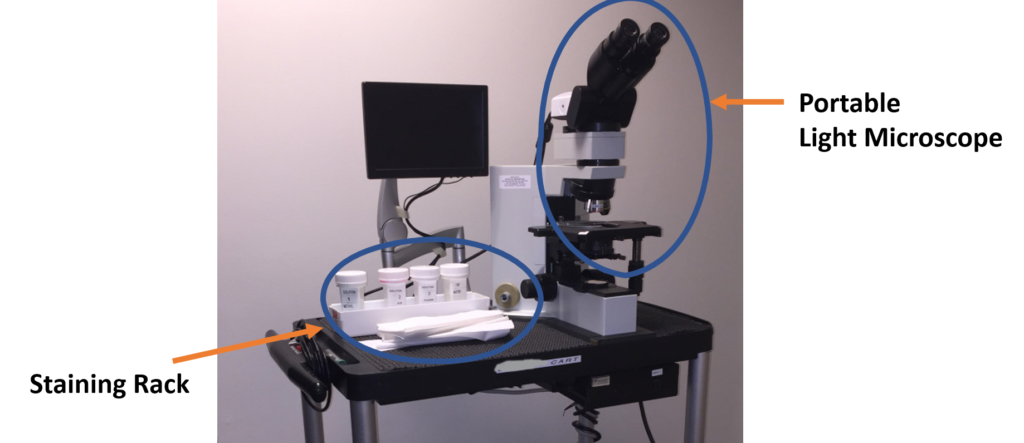
The Equipment: The ROSE Cart
All we need is a countertop and an electrical outlet. The example shows a portable light microscope (sometimes connected to a screen for telecytopathology) and a staining rack with both slides and our Diff quick (Romanowsky) stains for rapid staining of your samples.

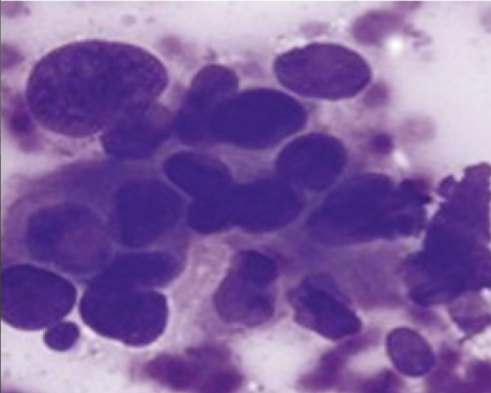
After we receive the needle, we place a drop on the slide and smear the sample. After it air dries we then stain using minimal material (“The Stains”), this is a manual process that takes at most 2 minutes (“The Staining”). The pathologist will then read the sample after mounting and coverslipping (“The Result”)

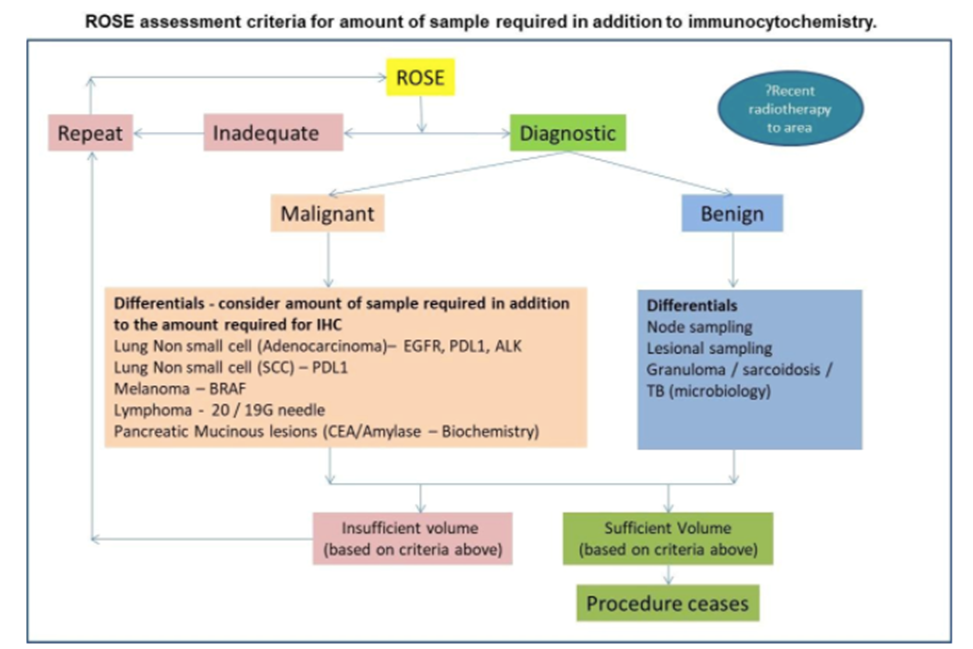
The Algorithm
We can then triage the specimen based on the pathologist’s call. If inadequate, additional samples can be taken, if lymphoma we can place in saline or RPMI for flow cytometry, if malignant we can place most of the specimen for cellblock preparation for immunohistochemistry and, if needed, we can give you a preliminary diagnosis so that you can get a head start on next steps for patient treatment.
Call us to discuss the feasibility of R.O.S.E. at your site today!
Additional Information and References
Cost: 700.00 TTD per pass, 300.00 TTD for any subsequent pass
Requisition forms: Available at globalpathologysolutions.com
- Glinski et al. Cytopathology30, 164–172 (2019)
- https://www.eurocytology.eu/en/course/1875
- https://onlinelibrary.wiley.com/doi/toc/10.1111/(ISSN)1365-2303.rapid_on-site_evaluation_of_cytological_samples
- Crothers BA, Tench WD, Schwartz MR, et al. Guidelines for the reporting of nongynecologic, cytopathology specimens. Archives of Pathology and Laboratory Medicine. 2009;133:1743‐56.
- Gupta PK: Progression from on‐site to point‐of‐care fine needle aspiration service: Opportunities and challenges. CytoJournal 2010;7:6.
- Collins BT, Chen AC, Wang JF, Bernadt CT, Sanati S. Improved Laboratory Resource Utilization and Patient Care with the Use of Rapid On‐Site Evaluation for Endobronchial Ultrasound Fine Needle Aspiration Biopsy. Cancer Cytopathology. 2013 October 121(10): 544‐551.
- Pantanowitz L, Wiley CA, Demetris A, et al. Experience with multimodality telepathology at the University of Pittsburgh Medical Center. J Pathol Inform. 2012;3:45.
- Burlingame O, Kessé K, Silverman S, Cibas E. On‐site adequacy evaluations performed by cytotechnologists: correlation with final interpretations of 5241 image‐guided fine‐needle aspiration biopsies. Cancer Cytopathology. 2012 Jun 25; 120(3):177‐184.
- Collins BT, Murad FM, Wang JF, Bernadt CT. Rapid on‐site evaluation for endoscopic ultrasound‐guided fine‐needle biopsy of the pancreas decreases the incidence of repeat biopsy procedures. Cancer Cytopathol. 2013;121:518–524.


